Learn about the electrochemistry in the batteries that power many of the devices you use every day.
Picture a world without lithium-ion batteries (often called Li-ion batteries or LIBs). Need help? Mobile devices wouldn’t look the way they do now. Picture huge, heavy cell phones and laptops. Also picture that both of these things are so expensive that only very rich people can afford them. What you are picturing is the 1980s. Scary, isn’t it?
Lithium-ion batteries have become a huge part of our mobile culture. They provide power to much of the technology that our society uses.
What are the parts of a lithium-ion battery?
A battery is made up of several individual cells that are connected to one another. Each cell contains three main parts: a positive electrode (a cathode), a negative electrode (an anode) and a liquid electrolyte.

Just like alkaline dry cell batteries, such as the ones used in clocks and TV remote controls, lithium-ion batteries provide power through the movement of ions. Lithium is extremely reactive in its elemental form. That’s why lithium-ion batteries don’t use elemental lithium. Instead, lithium-ion batteries typically contain a lithium-metal oxide, such as lithium-cobalt oxide (LiCoO2). This supplies the lithium-ions. Lithium-metal oxides are used in the cathode and lithium-carbon compounds are used in the anode. These materials are used because they allow for intercalation. Intercalation means that the molecules are able to insert something into them. In this case, the electrodes are able to have lithium-ions move easily in and out of their structures.
What is the chemistry involved in lithium-ion batteries?
Inside a lithium-ion battery, oxidation-reduction (Redox) reactions take place.
Reduction takes place at the cathode. There, cobalt oxide combines with lithium ions to form lithium-cobalt oxide (LiCoO2). The half-reaction is:
CoO2 + Li+ + e– → LiCoO2
Oxidation takes place at the anode. There, the graphite intercalation compound LiC6 forms graphite (C6) and lithium ions. The half-reaction is:
LiC6 → C6 + Li+ + e–
Here is the full reaction (left to right = discharging, right to left = charging):
LiC6 + CoO2 ⇄ C6 + LiCoO2
How does recharging a lithium-ion battery work?
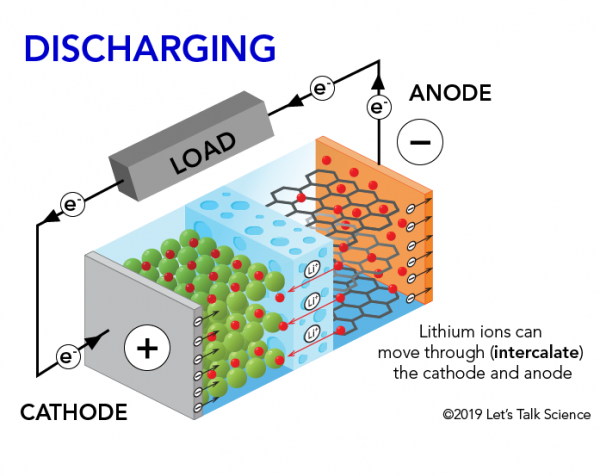
When the lithium-ion battery in your mobile phone is powering it, positively charged lithium ions (Li+) move from the negative anode to the positive cathode. They do this by moving through the electrolyte until they reach the positive electrode. There, they are deposited. The electrons, on the other hand, move from the anode to the cathode.
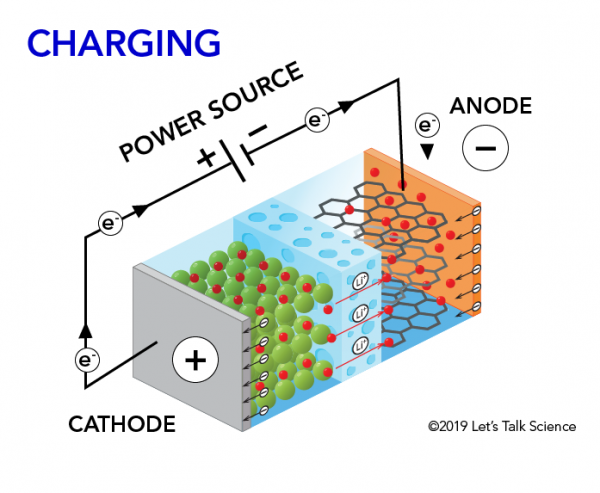
When you charge a lithium-ion battery, the exact opposite process happens. The lithium ions move back from the cathode to the anode. The electrons move from the anode to the cathode.
As long as lithium ions are making the trek from one electrode to another, there is a constant flow of electrons. This provides the energy to keep your device running. Since this cycle can be repeated hundreds of times, this type of battery is rechargeable.
What makes lithium-ion batteries good for mobile technologies?
It’s simple. lithium-ion batteries have the highest charge density of any comparable system. This means they can give you a ton of energy without being very heavy.
This is for two reasons. First, lithium is the most electropositive element. Electropositivity is a measure of how easily an element can donate electrons to produce positive ions. In other words, it’s a measure of how easily an element can produce energy. Lithium loses electrons very easily. This means it can easily produce a lot of energy.
Lithium is also the lightest of all metals. As you’ve learned, intercalation materials are used as electrodes in lithium-ion batteries instead of actual lithium metal. Still, these batteries weigh much less than other types of batteries that use metals like lead or nickel.
Are there any risks with using lithium-ion batteries?
While these batteries are pretty impressive, they do have their downsides. The biggest complaint is that they wear out fairly quickly whether you use them or not. A typical lithium-ion battery will last about 2-3 years before it has to be replaced. That can get expensive! The production and disposal of lithium-ion batteries also has a big impact on the environment, so the longer those batteries can last the better.
As you learned, lithium is extremely reactive. When manufacturers make lithium-ion batteries, they have to take certain precautions so that the batteries are safe to use. However, you may have heard of some electronics, such as laptops or cell phones, bursting into flames because of their batteries. While this might be a good excuse for not handing in your English essay on time, it’s a pretty dangerous situation. For safety reasons, lithium-ion batteries include a separator. This prevents the electrodes of the battery’s cells from touching each other. But if this separator gets ripped or damaged, the electrodes can touch. This can cause a huge build-up of heat. If this build-up of heat produces a spark, the highly flammable electrolyte can catch on fire.
Once there are flames in one cell, they can quickly spread to others. And before you know it, your laptop is a pool of melted plastic. A build-up of heat can also cause the pressure in your laptop to rise very quickly and BOOM!
source : https://letstalkscience.ca





